The success of dry eye disease (DED) treatment once relied heavily on the patient’s compliance to their prescribed treatments(s) when they left the exam room. With the dawn of in-office treatments, patients are more our partners in their care today than ever before. This helps with compliance to their prescribed treatments.
In this article, we discuss these in-office treatment options.
AMNIOTIC MEMBRANES
This regenerative layer of tissue aids in corneal re-epithelialization and provides apoptosis of pro-inflammatory cells in persistent keratitis (Figure 1). Additionally, amniotic membranes assist in anti-scarring, anti-angiogenesis and serve as a scaffold for limbal stem cells. This is especially helpful in conditions, such as limbal stem cell deficiency and graft-versus-host disease.
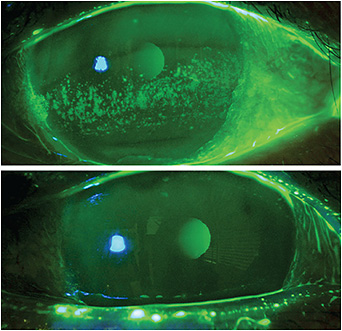
Figure 1. Amniotic membranes aid in corneal re-epithelialization and provide apoptosis of pro-inflammatory cells in persistent keratitis.Figure 1 courtesy of Dr. Kataria.
Two types of amniotic membrane are available for ophthalmological use: dehydrated and cryopreserved.
AUTOLOGOUS SERUM EYE DROPS
Derived from blood components that mimic the biological and biochemical properties of the natural tear film, autologous serum eye drops possess natural healing factors, such as growth factors and cytokines, that assist in corneal and conjunctival healing. Optometrists may work with local labs to facilitate blood draws and preparation or use Vitaltears.org. In some states, ODs can perform blood draws in-office, so it makes sense to check state scope-of-practice laws.
Autologous serum eye drop concentrations can vary between 20% to 100%, with typical starting doses at 20% to 40%, and a frequency of dosing between four to six times a day for three to six months. Once ocular surface health improvement is noted in DED signs and symptoms, frequency can be tapered accordingly.
INTENSE PULSED LIGHT
Intense pulsed light (IPL) can treat meibomian gland dysfunction (MGD). Specifically, it uses light energy to obliterate abnormal telangiectatic blood vessels that are a reservoir of inflammatory mediators. The result is the removal of a significant source of inflammation from the eyelids and meibomian glands. Overtime, IPL treatments improve the expressibility and quality of the meibum. Beyond reducing inflammation, IPL is shown to eradicate Demodex, soften meibum, and improve meibomian gland structure and function over time.
Patients who have rosacea-related ocular surface disease are ideal patients for IPL treatments (Figure 2). This is because IPL uses specific wavelengths of light to target the inflammatory telangiectatic blood vessels in rosacea and help diminish them over time, thereby reducing inflammation on the skin and around the meibomian glands. That said, treatments are limited to Fitzpatrick skin types I-V in all patients. (See bit.ly/FitzSkin .)
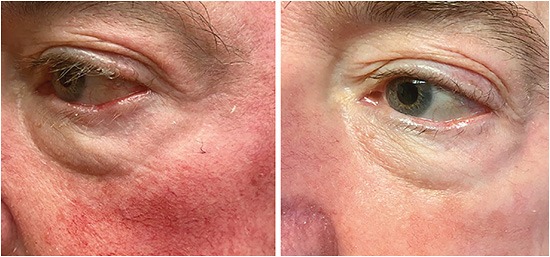
Figure 2. Intense pulsed light is an ideal treatment for patients who have rosacea-related ocular surface disease because the treatment uses specific wavelengths of light to help diminish telangiectatic blood vessels over time.Figure 2 courtesy of Dr. Madan.
Additionally, studies support combining IPL with lid debridement and meibomian gland expression to further help improve DED symptoms and meibomian gland function.3
LOW-LEVEL LIGHT THERAPY
Low level light therapy (LLLT) employs light-emitting facemasks of specific wavelengths of light to induce photobiomodulation in the face and periorbital tissue.4 This makes it another treatment option for MGD. LLLT is athermal, so it can be applied to the upper eyelids sans laser grade corneal shields. Combining IPL and LLLT can have additive effects to improve MGD.5
Choosing one form of light therapy over another is based on the optometrist’s preference.
MEIBOMIAN GLAND EXPRESSION
This is the manual expression of the meibomian glands via forceps. Cold meibomian gland expression is generally not recommended due to the delicate nature of the meibomian gland acinar cells. Cold expression of the glands with gentle pressure is used only as a diagnostic tool to assess the meibum quality and quantity. It is generally recommended to heat the glands prior to expression with forceps.
MEIBOMIAN GLAND PROBING
This treatment may be a viable solution to widen the meibomian glands to assist in meibum release when intraductal fibrosis and meibomian gland stenosis are suspected. A study reveals that 24 out of 25 patients reported immediate improvement in their DED symptoms after meibomian gland probing.6 Of note: Probing is a technique not widely performed, especially in a primary care OD setting, likely because the scope-of-practice restrictions are involved. Also, probing is typically thought of when duct scarring is suspected, or significant pressure is encountered, making the number of subjects significantly less in most meibomian gland probing studies.
With the help of local anesthesia, a probe (available in various lengths) is inserted into the meibomian gland orifice to release intraductal pressure and facilitate meibum excretion. A recent literature review suggests that meibomian gland probing may be particularly efficacious when combined with IPL, corticosteroids, and conventional treatments for MGD, including meibomian gland expression.7
MICROBLEPHAROEXFOLIATION
One of the challenges of overseeing DED can be managing blepharitis. Both Staphylococcal and Demodex blepharitis require ongoing and diligent at-home lid hygiene. That said, in addition to the challenge of patient compliance, patient dexterity and the required techniques of at-home lid hygiene can be a hindrance to success. This is where in-office microblepharoexfoliation comes in.
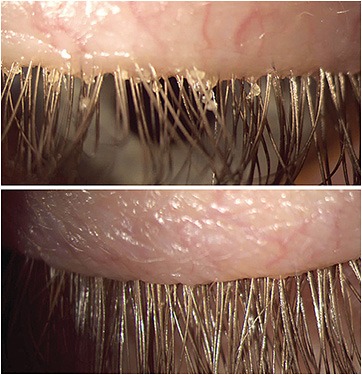
Specifically, it cleans accumulated bacterial biofilms from the eyelid and lash margins manually, (Figure 3) or mechanically (a motorized brush, like an electric toothbrush), acting as a support to prescribed home care and resulting in the improved symptoms and signs of DED.

Figure 3. Microblepharoexfoliation cleans accumulated bacterial biofilms from the eyelid and lash margins manually or mechanically (a motorized brush, like an electric toothbrush), resulting in improved DED symptoms and signs.Figure 3 courtesy of Dr. Madan.
OPHTHALMIC INSERTS
This is placed by the patient into their inferior fornix, commonly once a day, as a means of achieving preservative-free lubrication.
One study shows ophthalmic inserts greatly decreased both the symptoms and signs of moderate-to-severe DED. Additionally, patients reported a major improvement in quality of life, according to Ocular Surface Disease Index scores.8
PLATELET-RICH PLASMA (PRP)
This treatment for advanced DED contains a high concentration of growth factors due to the presence of platelets, which are eliminated in autologous serum eye drops. Platelets play a key role in the wound-healing process, which is needed for damaged tissue recovery.
Platelet-rich plasma has also been successfully used in the treatment of recalcitrant DED and in dormant corneal ulcers and recurrent corneal erosions.9,10 Another study shows that platelet-rich plasma can help increase corneal nerve density, which is lost in chronic DED.11
PUNCTAL PLUGS
These can be used as a maintenance treatment to conserve tear volume once DED-caused ocular surface inflammation is controlled. They are particularly beneficial in patients whose DED has a significant aqueous-deficient component and as a first-line therapy for neurotrophic keratitis.12
Punctal plugs can be inserted in-office with a punctal dilator and forceps. They are typically placed in the lower puncta initially; all four puncta are used in more severe cases.
THERMAL TREATMENT
Thermal treatment can be used for MGD patients who cannot achieve symptom relief from an at-home regiment of warm compresses and lid hygiene alone. There are different types of thermal devices. For example, one uses activators and heats the meibum and enables gland expression via pulsation. One heats the meibum and provides gland expression in one step through a handheld device. Further, one provides activators connected to a hub that provides an ongoing flow of heat to melt the meibum.
Each procedure allows the patient to return to their normal activities with minimal, if any, downtime. We do recommend that patients resume their current regimen of daily warm compresses and lid hygiene afterwards to best maintain their treatment effect.
Thermal treatment can be repeated as needed, but we of-ten recommend every six to 12 months, depending on the patient’s severity of MGD and DED symptoms.
PARTNERING WITH PATIENTS
In-office DED treatments can be an excellent adjunct to prescribed at-home DED treatments. Also, they enable a partnership among our DED patients and ourselves when it comes to their care, aiding in adherence to their prescribed treatments. In addition, offering such treatments can be a practice builder, as they can differentiate the optometrist from eye care providers who do not offer them. OM
REFERENCES
Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017;18(2):193-204. doi: 10.1007/s10561-017-9618-5.
Suwal A, Hao JL, Zhou DD, Liu XF, Suwal R, Lu CW. Use of Intense Pulsed Light to Mitigate Meibomian Gland Dysfunction for Dry Eye Disease. Int J Med Sci. 2020;17(10):1385-1392. doi: 10.7150/ijms.44288.
Moon SY, Han SA, Kwon HJ, Park SY, et al. Effects of lid debris debridement combined with meibomian gland expression on the ocular surface. MMP-9 levels and clinical outcomes in moderate and severe meibomian gland dysfunction. BMC Ophthalmol. 2021;21(1):175. doi: 10.1186/s12886-021-01926-2.
Solomos L, Bouthour W, Malclès A, Thumann G, Massa H. Meibomian Gland Dysfunction: Intense Pulsed Light Therapy in Combination with Low-Level Light Therapy as Rescue Treatment. Medicina (Kaunas). 2021;57(6):619. doi: 10.3390/medicina57060619.
Stonecipher K, Abell TG, Chotiner B, Chotiner E, Potvin R. Combined low level light therapy and intense pulsed light therapy for the treatment of meibomian gland dysfunction. Clin Ophthalmol. 2019;13:993-999. doi: 10.2147/OPTH.S213664.
Maskin SL. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea. 2010 Oct;29(10):1145-52.
Magno M, Moschowits E, Arita R, Vehof J, Utheim TP. Intraductal meibomian gland probing and its efficacy in the treatment of meibomian gland dysfunction. Surv Ophthalmol. 2021;66(4):612-622. doi: 10.1016/j.survophthal.2020.11.005.
McDonald M, D’Aversa G, Perry HD, Wittpenn JR, Donnenfeld ED, Nelinson DS. Hydroxypropyl cellulose ophthalmic inserts (Lacrisert) reduce the signs and symptoms of dry eye syndrome and improve patient quality of life. Trans Am Ophthalmol Soc. 2009;107:214-21.
Alio JL, Abad M, Artola A, Rodriguez-Prats JL, Pastor S, Ruiz-Colecha J. Use of autologous platelet-rich plasma in the treatment of dormant corneal ulcers. Ophthalmology. 2007;114:1286-1293. doi: 10.1016/j.ophtha.2006.10.044.
Lee JH, Kim MJ, Ha SW, Kim HK. Autologous Platelet-rich Plasma Eye Drops in the Treatment of Recurrent Corneal Erosions. Korean J Ophthalmol. 2016;30(2):101-107. doi: 10.3341/kjo.2016.30.2.101.
Fea AM, Aragno V, Testa V, et al. The Effect of Autologous Platelet Lysate Eye Drops: An In Vivo Confocal Microscopy Study. Biomed Res Int. 2016;2016:8406832. doi: 10.1155/2016/8406832.
Tong L, Zhou L, Beuerman R, Simonyi S, Simonyi S, Hollander DA, Stern ME. Effects of punctal occlusion on global tear proteins in patients with dry eye. Ocul Surf. 2017;15(4):736-741. doi: 10.1016/j.jtos.2017.04.002.
DR. KATARIA is a graduate of the New England College of Optometry. After completing a residency in Primary Care and Ocular Disease, she relocated to Los Angeles, where she practices in a medical office mostly managing dry eye disease and glaucoma. She discloses a financial relationship with Tarsus Pharmaceuticals.
DR. MADAN graduated from the Pacific University College of Optometry. She completed a residency in ocular disease and surgical co-management at the Eye Center of Texas in Houston. She practices in Vancouver, BC, and focuses on the utilization of innovative treatments for advanced dry eye disease. She discloses financial relationships with Lumenis, SunPharma, and Labitician.
Source
https://optometricmanagement.com/issues/2022/july/consider-in-office-dry-eye-treatments/






